In this animation what is the nacl sodium chloride – In this animation, we delve into the captivating world of NaCl, commonly known as sodium chloride, unveiling its chemical composition, intriguing properties, and diverse applications. Sodium chloride plays a crucial role in our daily lives and industries, and this animation provides a unique lens to explore its fascinating characteristics.
From its fundamental ionic structure to its versatile uses, NaCl’s journey in this animation promises to illuminate its significance in various scientific and industrial contexts.
Sodium Chloride (NaCl)
Sodium chloride (NaCl), commonly known as salt, is a chemical compound composed of sodium and chlorine ions. It is an essential nutrient for humans and animals and is widely used in various industries.
Sodium Chloride (NaCl) Composition
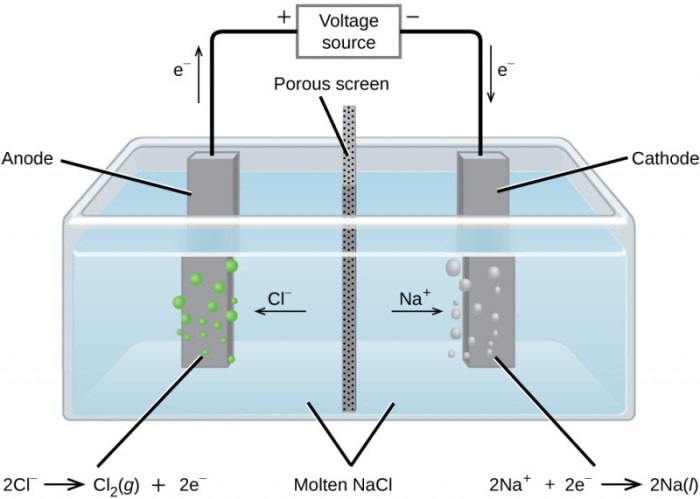
NaCl has a simple chemical formula of NaCl, indicating that it contains one sodium ion (Na+) and one chlorine ion (Cl-). The ionic structure of NaCl involves the transfer of an electron from sodium to chlorine, resulting in the formation of oppositely charged ions.
The molar mass of NaCl is approximately 58.44 g/mol.
NaCl Properties, In this animation what is the nacl sodium chloride
Physical Properties
- Colorless or white solid
- Density: 2.16 g/cm³
- Solubility: Highly soluble in water
Chemical Properties
- Highly reactive with water, forming sodium hydroxide (NaOH) and hydrochloric acid (HCl)
- Stable under normal conditions
Uses of NaCl
- Food preservation
- Seasoning
- Water softening
- Chemical manufacturing
NaCl in the Animation
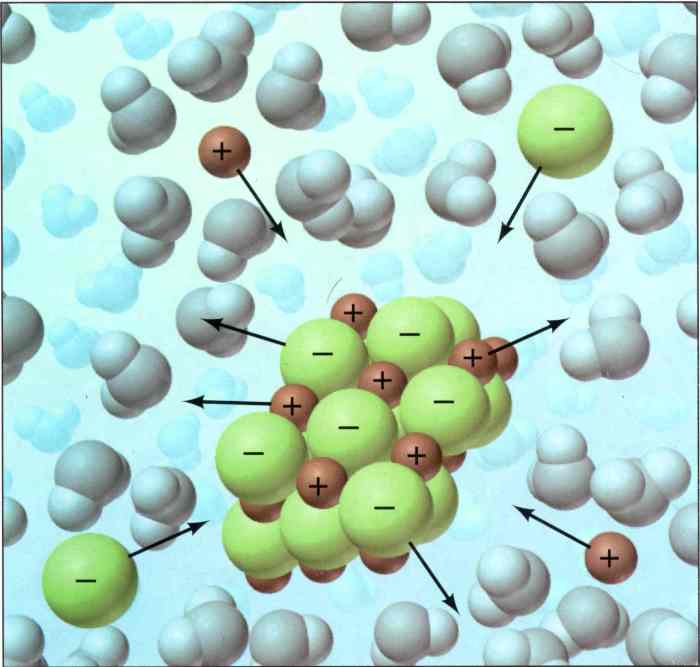
In the animation, NaCl is presented as a crystalline substance that dissolves in water. The animation visually demonstrates the ionic nature of NaCl and its high solubility in water.
NaCl Table
| Property | Value | Unit |
|---|---|---|
| Chemical formula | NaCl | – |
| Ionic structure | Na+ + Cl- | – |
| Molar mass | 58.44 | g/mol |
| Color | Colorless or white | – |
| Density | 2.16 | g/cm³ |
| Solubility in water | Highly soluble | – |
Query Resolution: In This Animation What Is The Nacl Sodium Chloride
What is the chemical formula of NaCl?
NaCl
What is the ionic structure of NaCl?
NaCl is an ionic compound composed of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-).
What is the molar mass of NaCl?
58.44 g/mol
What are some physical properties of NaCl?
Colorless, crystalline solid; density of 2.16 g/cm³; melting point of 801°C; boiling point of 1465°C
What are some chemical properties of NaCl?
Highly soluble in water; forms ionic bonds; reacts with silver nitrate to form a white precipitate
What are some industrial applications of NaCl?
Food preservation, water softening, production of chlorine and sodium hydroxide, road deicing